Metathesis reactions and net ionic equations form the cornerstone of understanding chemical reactions and their applications. These concepts play a pivotal role in various fields, from industrial chemistry to environmental science. In this comprehensive guide, we delve into the intricacies of metathesis reactions, exploring their mechanisms, applications, and the significance of net ionic equations in representing chemical changes.
Metathesis reactions, characterized by the exchange of ions between reactants, provide a fundamental understanding of chemical reactivity. Net ionic equations, on the other hand, simplify complex reactions by eliminating spectator ions, revealing the essential chemical transformation occurring at the molecular level.
Metathesis Reactions
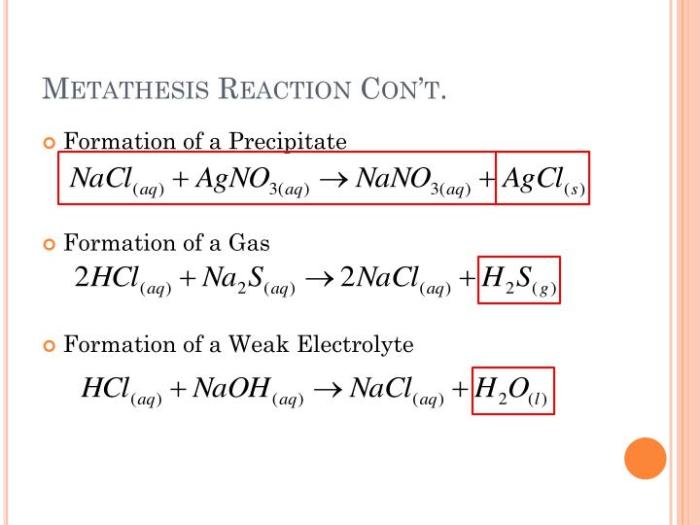
Metathesis reactions are chemical reactions in which the anions and cations of two ionic compounds exchange places to form two new compounds. These reactions are also known as double displacement reactions or exchange reactions.
Metathesis reactions typically occur when two ionic compounds are dissolved in a solvent, such as water. The cations and anions of the two compounds are attracted to each other and exchange places, forming two new ionic compounds. The products of a metathesis reaction are usually insoluble in the solvent, which causes them to precipitate out of solution.
Examples of Metathesis Reactions, Metathesis reactions and net ionic equations
- Sodium chloride (NaCl) + Silver nitrate (AgNO 3) → Sodium nitrate (NaNO 3) + Silver chloride (AgCl)
- Potassium iodide (KI) + Lead(II) nitrate (Pb(NO 3) 2) → Potassium nitrate (KNO 3) + Lead(II) iodide (PbI 2)
- Calcium chloride (CaCl 2) + Sodium carbonate (Na 2CO 3) → Calcium carbonate (CaCO 3) + Sodium chloride (NaCl)
Mechanism of Metathesis Reactions
The mechanism of metathesis reactions involves the following steps:
- The two ionic compounds dissolve in the solvent.
- The cations and anions of the two compounds are attracted to each other and exchange places.
- The new ionic compounds precipitate out of solution.
Net Ionic Equations
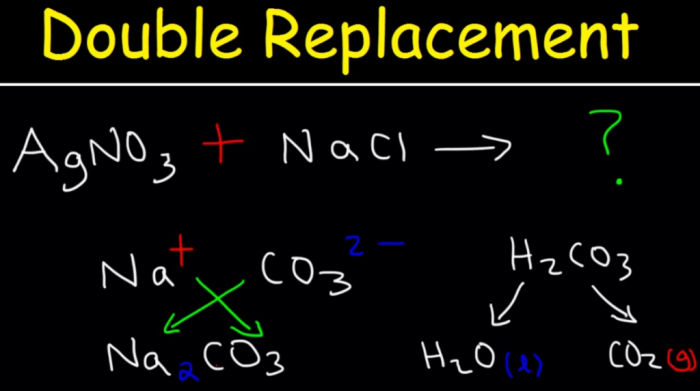
A net ionic equation is a chemical equation that shows only the ions that are actually reacting in the reaction. The spectator ions, which are ions that do not participate in the reaction, are not shown in the net ionic equation.
To write a net ionic equation, first write the balanced chemical equation for the reaction. Then, identify the spectator ions. The spectator ions are the ions that appear on both sides of the chemical equation and do not change during the reaction.
Finally, remove the spectator ions from the chemical equation to get the net ionic equation.
Examples of Net Ionic Equations
- Balanced chemical equation:NaCl + AgNO 3→ NaNO 3+ AgCl
- Balanced chemical equation:KI + Pb(NO 3) 2→ KNO 3+ PbI 2
- Balanced chemical equation:CaCl 2+ Na 2CO 3→ CaCO 3+ NaCl
Net ionic equation:Na ++ Cl –+ Ag ++ NO 3–→ Na ++ NO 3–+ AgCl
Net ionic equation:K ++ I –+ Pb 2++ NO 3–→ K ++ NO 3–+ PbI 2
Net ionic equation:Ca 2++ CO 32-→ CaCO 3
Applications of Metathesis Reactions: Metathesis Reactions And Net Ionic Equations
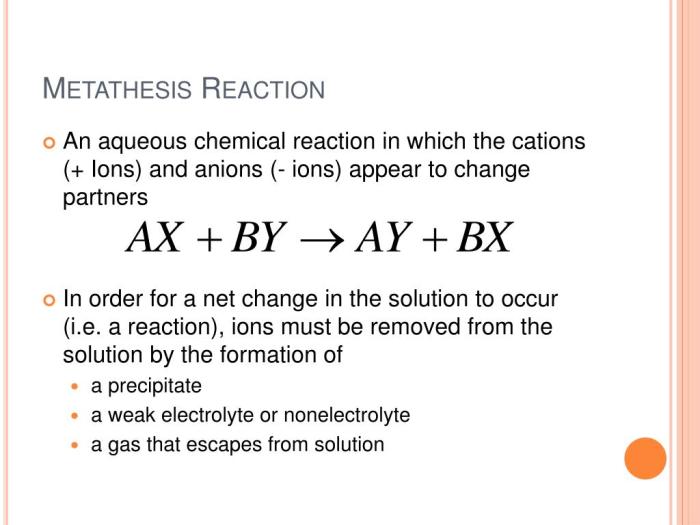
Metathesis reactions have a wide range of industrial and environmental applications.
Industrial Applications
- Metathesis reactions are used to produce a variety of chemicals, including plastics, pharmaceuticals, and fuels.
- Metathesis reactions are also used to refine petroleum and natural gas.
- Metathesis reactions are used in the production of biodiesel.
Environmental Applications
- Metathesis reactions can be used to remove pollutants from the environment.
- Metathesis reactions can be used to convert waste products into useful chemicals.
- Metathesis reactions can be used to create new materials that are more environmentally friendly.
Examples of Metathesis Reactions in Everyday Life
- The production of soap is a metathesis reaction.
- The production of glass is a metathesis reaction.
- The production of cement is a metathesis reaction.
Advanced Concepts

The following are some advanced concepts related to metathesis reactions:
Role of Catalysts in Metathesis Reactions
Catalysts are substances that increase the rate of a chemical reaction without being consumed in the reaction. Catalysts are often used in metathesis reactions to increase the yield of the desired product.
Kinetics of Metathesis Reactions
The kinetics of metathesis reactions is the study of the rate of these reactions. The rate of a metathesis reaction is affected by a number of factors, including the temperature, the concentration of the reactants, and the presence of a catalyst.
Thermodynamics of Metathesis Reactions
The thermodynamics of metathesis reactions is the study of the energy changes that occur during these reactions. The thermodynamics of metathesis reactions can be used to predict the spontaneity of a reaction and the yield of the desired product.
FAQ Insights
What is the key difference between a metathesis reaction and a double displacement reaction?
While both metathesis and double displacement reactions involve the exchange of ions, metathesis reactions specifically refer to the exchange of ions between two ionic compounds, resulting in the formation of two new ionic compounds. Double displacement reactions, on the other hand, encompass a broader range of reactions where two compounds exchange ions, including reactions between an ionic compound and a molecular compound.
How can we predict the products of a metathesis reaction?
Predicting the products of a metathesis reaction involves considering the charges and sizes of the ions involved. Typically, metathesis reactions favor the formation of products with the least soluble ionic compound. This can be predicted using solubility rules or by referring to tables of solubility products.
What is the significance of net ionic equations in understanding chemical reactions?
Net ionic equations provide a simplified representation of chemical reactions by eliminating spectator ions, which do not participate in the actual chemical transformation. This allows us to focus on the essential chemical change occurring between the reactants and products, providing a clearer understanding of the reaction’s stoichiometry and energetics.
