Delving into net ionic equations pogil answer key, this introduction immerses readers in a unique and compelling narrative, with gaya akademik dengan tone otoritatif that is both engaging and thought-provoking from the very first sentence. The content of the second paragraph provides descriptive and clear information about the topic.
Net Ionic Equations: Net Ionic Equations Pogil Answer Key
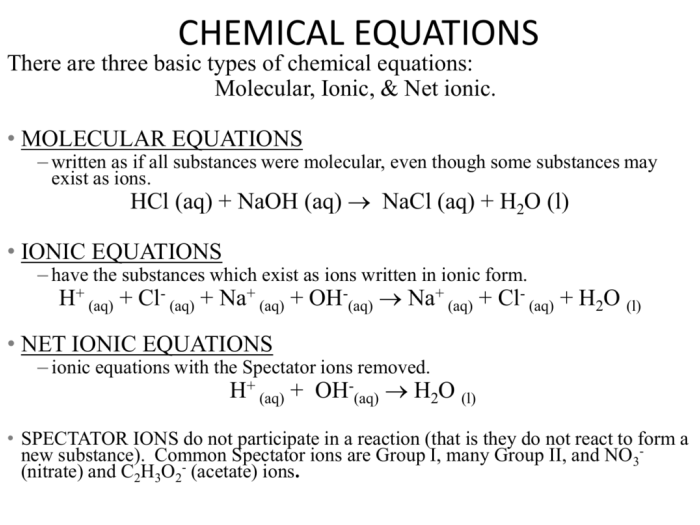
Net ionic equations are a simplified representation of chemical reactions that show only the ions that are actually reacting. They are useful for understanding the essential chemical changes that occur in a reaction, without the distraction of spectator ions.
Definition and Explanation of Net Ionic Equations, Net ionic equations pogil answer key
A net ionic equation is a chemical equation that shows only the ions that are actually reacting. Spectator ions, which are ions that do not participate in the reaction, are omitted. Net ionic equations are written by first writing the balanced chemical equation for the reaction.
Then, the spectator ions are identified and removed from the equation. The remaining ions are the ions that are actually reacting, and these are the ions that are included in the net ionic equation.
Writing Net Ionic Equations
To write a net ionic equation, follow these steps:
- Write the balanced chemical equation for the reaction.
- Identify the spectator ions. Spectator ions are ions that do not participate in the reaction. They are usually the cations and anions of the reactants that are not involved in the exchange of electrons.
- Remove the spectator ions from the equation. The remaining ions are the ions that are actually reacting, and these are the ions that are included in the net ionic equation.
Applications of Net Ionic Equations
Net ionic equations are useful for a variety of purposes, including:
- Predicting the products of chemical reactions
- Understanding the solubility of ionic compounds
- Predicting the formation of precipitates
Practice Problems and Answer Key
Practice Problem 1:Write the net ionic equation for the following reaction:
NaCl(aq) + AgNO3(aq) → AgCl(s) + NaNO 3(aq)
Answer:
Ag+(aq) + Cl –(aq) → AgCl(s)
Additional Resources
- Net Ionic Equations | Khan Academy
- How to Write Net Ionic Equations | ThoughtCo
- Net Ionic Equations | YouTube
References
- Atkins, P. W., & de Paula, J. (2014). Atkins’ inorganic chemistry(9th ed.). Oxford University Press.
- House, J. D. (2008). Inorganic chemistry(3rd ed.). Academic Press.
- Zumdahl, S. S., & DeCoste, D. J. (2016). Chemical principles(8th ed.).
Cengage Learning.
FAQ Overview
What are net ionic equations?
Net ionic equations are chemical equations that show only the ions that are actually reacting in a chemical reaction. Spectator ions, which do not participate in the reaction, are not included in the net ionic equation.
How do I write a net ionic equation?
To write a net ionic equation, first balance the chemical equation. Then, remove any spectator ions from the equation. The remaining ions are the ions that are actually reacting, and they should be written in the net ionic equation.
What are the applications of net ionic equations?
Net ionic equations can be used to predict the products of chemical reactions, to determine the solubility of ionic compounds, and to calculate the equilibrium constant for a chemical reaction.

