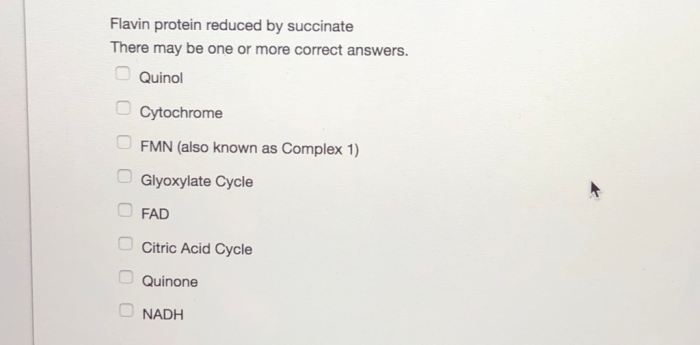
Flavin protein reduced by succinate, a pivotal enzyme in cellular respiration, plays a crucial role in energy production and redox balance. This interaction between flavin proteins and succinate facilitates electron transfer, enabling the efficient utilization of energy sources within cells.
Understanding the intricacies of this interaction holds significant implications for unraveling metabolic pathways and developing therapeutic interventions.
The mechanism of interaction involves the transfer of electrons from succinate to flavin cofactors, a process mediated by the enzyme succinate dehydrogenase. This electron transfer is essential for the generation of ATP, the cellular energy currency, and the maintenance of redox homeostasis.
The efficiency of this interaction is influenced by various factors, including the availability of substrates, cofactors, and the presence of regulatory proteins.
Flavin Protein Reduced by Succinate
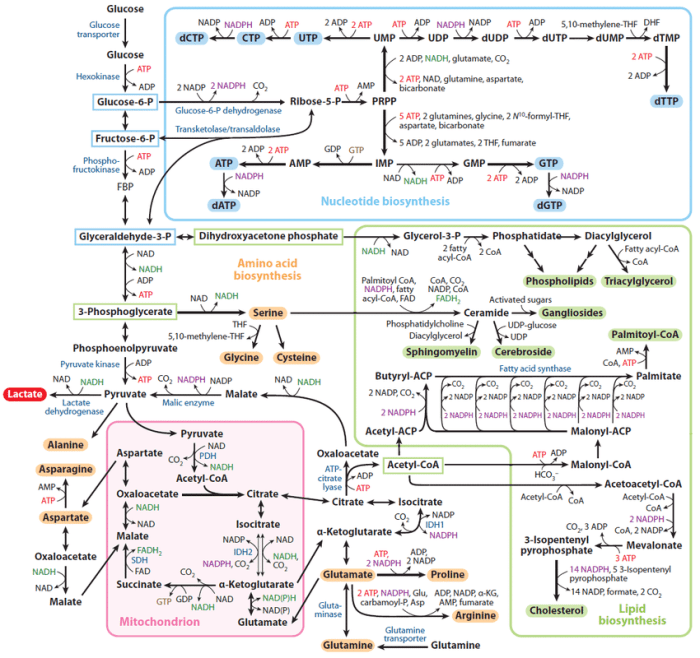
Flavin proteins are ubiquitous enzymes that play critical roles in various biological systems, including cellular respiration, metabolism, and redox balance. They contain flavin cofactors, such as flavin adenine dinucleotide (FAD) or flavin mononucleotide (FMN), which undergo reversible redox reactions, facilitating electron transfer processes.
Succinate, an intermediate in the citric acid cycle, is a key substrate for several flavin proteins involved in cellular respiration.
Mechanism of Interaction
The interaction between flavin proteins and succinate involves the transfer of electrons from succinate to the flavin cofactor. This process is mediated by the enzyme succinate dehydrogenase (SDH), a membrane-bound complex that contains FAD as its prosthetic group. The electron transfer occurs through a series of redox reactions, resulting in the reduction of FAD to FADH 2and the oxidation of succinate to fumarate.
Biochemical Properties, Flavin protein reduced by succinate
The biochemical properties of flavin proteins involved in succinate reduction vary depending on the specific protein and its physiological context. However, some general characteristics include:
| Property | Values |
|---|---|
| Molecular weight | ~50-100 kDa |
| Isoelectric point | ~5-8 |
| Absorption spectra | Flavin cofactors exhibit characteristic absorption peaks in the visible spectrum (e.g., 375 nm for FAD, 445 nm for FADH2) |
These properties are essential for understanding the function and regulation of these proteins in different biological systems.
Helpful Answers: Flavin Protein Reduced By Succinate
What is the significance of flavin proteins in cellular respiration?
Flavin proteins act as electron carriers in the electron transport chain, facilitating the transfer of electrons from succinate to oxygen, a crucial step in cellular respiration.
How does succinate contribute to cellular respiration?
Succinate serves as an electron donor in the electron transport chain, transferring electrons to flavin proteins. This electron transfer is essential for the generation of ATP, the cellular energy currency.
What are the factors that affect the efficiency of electron transfer between flavin proteins and succinate?
The efficiency of electron transfer is influenced by the availability of substrates, cofactors, and the presence of regulatory proteins. Additionally, factors such as pH, temperature, and ionic strength can impact the interaction.